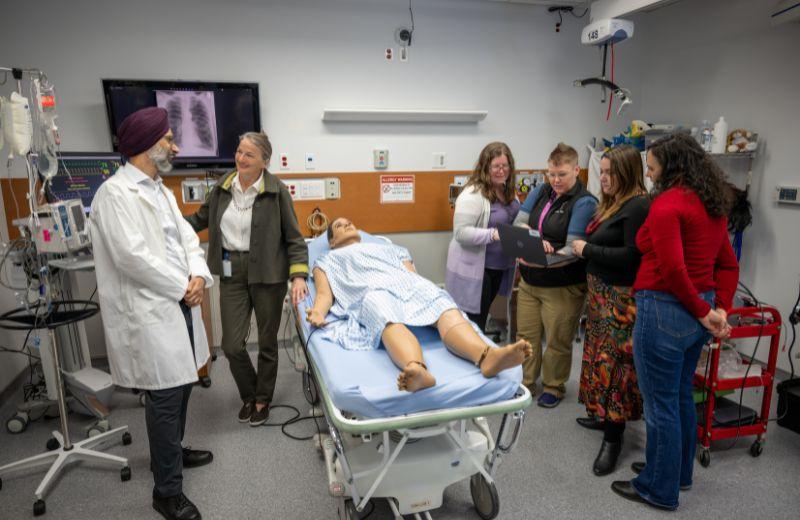
The Northern Centre for Clinical Research (NCCR) launched its first clinical trial this year at the University Hospital of Northern British Columbia (UHNBC) in Prince George. The NCCR is a collaborative initiative between University of British Columbia (UBC) Faculty of Medicine, the University of Northern BC (UNBC) and Northern Health (NH), with the mission to enhance the capacity and cohesion of the clinical and life sciences research programs in the North, creating strategic opportunities to share resources among UBC faculty, NH, and UNBC in collaboration with local partners.
The clinical trial is part of an international study based out of Oxford Population Health called EASi-KIDNEY, which aims to recruit 11,000 people with chronic kidney disease to assess the effectiveness of a new drug called vicadrostat (also known as BI 690517) in reducing risk of kidney disease progression, hospitalization for heart failure, and/or death from cardiovascular disease in people with chronic kidney disease. This is a multi-year study with results expected in 2028/29.

“Northern Health has been working hard over the last couple of years to build a team and infrastructure that supports clinical research for people in the North,” said Julia Bickford, NH Executive Director of Research Innovation & Evaluation. “Implementing our Standard Operating Procedures, Clinical Trials Management System, and building relationships with related departments, like pharmacy and diagnostics, have been a huge undertaking. It is very exciting to see all this work result in greater access to clinical trials for patients in the North.”
She added, “Through the dedication of investigators, like Dr. Anurag Singh, and the extraordinary teams that have come together to support clinical research in NH and the NCCR, we are able to provide access to treatment options that would otherwise require travel to a major city. This is a positive step forward for health care and research in Northern BC.”
NH contributed to the clinical trial by providing the physical space for the trials at UHNBC, funding three full-time positions (a regional research manager, a clinical research nurse coordinator, and a quality management coordinator), and a part-time administrative assistant. NH also provided lab support for the clinical trial.
NCCR staff were integral in making the clinical trial launch happen by creating and implementing Standard Operating Procedures (SOPs) and implementing the Clinical Trial Management System.
“The EASi-KIDNEY trial marks a significant milestone for NH and the NCCR, as it’s the first Phase 3 interventional trial in our region initiated by NH and reflects our growing capacity to support high-quality research rooted in Northern realities,” said Dr. Anurag Singh, Principal Investigator of the clinical trial. “This study offers people living with chronic kidney disease in rural and Indigenous communities a rare opportunity to participate in cutting-edge international research in our region.” He continued, “It brings access to a promising treatment to help slow disease progression and improve long-term outcomes. Just as importantly, it ensures that the unique experiences of Northern patients are represented in the evidence shaping kidney care worldwide.”
Learn more
Learn more about this and other research going on at NH on our webpage.












Comments
Information about the specific needs of the research trial can be found here: https://www.easikidney.org/. The researchers involved will be able to outline what is needed from participants and asses whether you would need to travel or not in order to participate.